Intellectual Property and Data Access
Applicants will retain ownership of any intellectual property that they bring to the program and may be offered licensing rights on inventions generated by the government or its agents in furtherance of the program, depending on the stage of development.
NCI Patent Rights and Licensing Agreement Terms
for the NExT Program
The NCI has a variety of agreement types outlining the different patent and licensing mechanisms applicable to studies of third-party agents in the NExT Program. One or more of the following agreements may be applied depending on the stage at which the project entered the NExT pipeline and the inventions generated as the project advanced. The NCI will work with NCI Technology Transfer Center to determine the appropriate agreement for studies approved by the NExT Program.
NExT Pipeline — Agreement Types by Project Phase
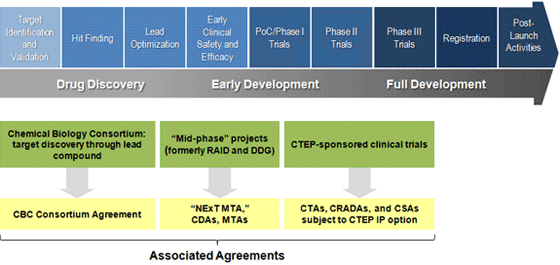
CBC Participants Agreement (Discovery Projects):
Work conducted by Basic Ordering Agreement (BOA) holders and other signatories of the CBC Participants Agreement is subject to the Bayh-Dole Act (35 U.S.C. § 200, et. seq.); in addition, all CBC members are expected to follow the principles outlined in the CBC Participants Agreement and the Good Faith Agreement.
NExT MTA (Mid-Phase Projects):
Applicants whose projects enter the pipeline after the lead development stage but prior to clinical evaluation (including activities formerly known as RAID) are expected to execute a NExT MTA. The terms of this agreement will govern the collaboration between the NCI and the applicant on early development stage preclinical projects.
CTAs, CRADAs, and CSAs (Cancer Therapy Evaluation Program (CTEP)-Sponsored Clinical Trials):
Currently, the language for the management of patent rights under agreement with NCI for CTEP-sponsored clinical trials states that clinical trial institutions will grant the NExT Collaborator a paid-up non-exclusive research use license and offer the time-limited first option to negotiate a royalty-bearing license on any clinical trial institution inventions generated from the NExT Collaboration. For combination studies, all collaborators providing investigational agents for combination studies will receive a non-exclusive, royalty-free commercialization license to any inventions generated under the course of study that incorporate their agent. These agreements include the Clinical Trials Agreement (CTA), Cooperative Research and Development Agreement (CRADA), and Clinical Supply Agreement (CSA). More information regarding CTEP-sponsored clinical trials is available on the CTEP Web site.
NCI Employee Inventions:
The rights that NCI retains in employee inventions depend on the stage of development and the agreement negotiated between the NCI and the parties.
For early stage CBC agreements, most of the work will be conducted by contract and non-contract CBC participants as opposed to Federal laboratories. As such there will be few if any government inventions generated under these studies. Should conception or reduction to practice occur within the Federal government, the NCI will follow the licensing guidelines established by the NIH Office of Technology Transfer.
For mid stage NExT MTA projects, normally the NCI will not acquire intellectual property rights to inventions made by its employees using NExT Collaborator Materials under NExT unless the NExT Collaborator and the NCI mutually agree that to do so would be in the best interest of the NExT Collaborator and the invention's further development. The NCI will inform the NExT Collaborator of any such inventions, and, after consultation with the NExT Collaborator, the NCI will decide whether or not to file a patent application on any such invention. If the NCI does file a patent application, the NExT Collaborator will be given an opportunity to negotiate for a license in accordance with the procedures set forth in 37 CFR Part 404.
For late stage CTEP projects, the Collaborator will have licensing rights in any NCI employee subject invention as defined by the CRADA agreement between the NCI and the Collaborator. The licensing terms will be described in the model CRADA and will be substantially similar to the language found in the CTEP IP Option.
Contract Research Organizations (CROs):
The NCI will inform the NExT Collaborator of the results of the NCI contractor’s or subcontractor’s research using the NExT Collaborator’s Materials.
Inventions made by the CROs are subject to the Bayh-Dole Act; however, to encourage applicants to participate in the NExT Program, such NCI contractors and subcontractors have agreed to include, as a term and condition of their funding agreements, an agreement to offer to the NExT Collaborator a first option to negotiate a license to subject contractor or subcontractor inventions made using the NExT Collaborator’s Research Material.
Data Access
Data generated by NExT services are stored in highly secure, password-protected databases and file storage systems operating inside the NIH firewall. A user has to hold a NIH account and obtain appropriate approval to gain access to the databases. NIH accounts are only granted to those who have completed an NIH security awareness training course and account access is enforced with strict password criteria. Database access is granted on a “need-to-know” basis, accompanied by a further restraint that data are not to be shared with any individuals without direct access.





