Discovery
Pharmacodynamic Biomarkers
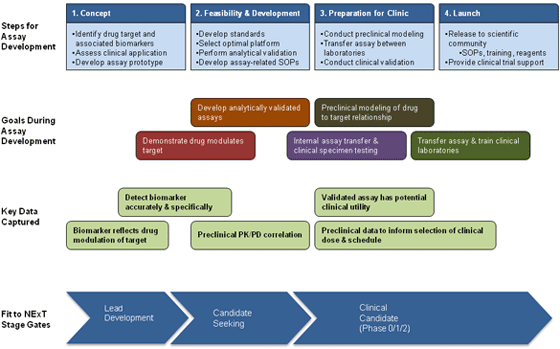
Pharmacodynamic (PD) biomarkers are molecular indicators of drug effect on the target in an organism. A PD biomarker can be used to examine the link between drug regimen, target effect, and biological tumor response. Coupling new drug development with focused PD biomarker measurements provides critical data to make informed, early go/no-go decisions, to select rational combinations of targeted agents, and to optimize schedules of combination drug regimens. Use of PD endpoints also enhances the rationality and hypothesis-testing power throughout drug development, from selection of lead compounds in preclinical models to first-in-human trials. Given these critical roles in delivering targeted therapy, the introduction of validated and reliable clinical PD assays is warranted in drug development.
Two biomarker programs, Imaging and Clinical Assay Laboratories, have been set up within the Division of Cancer Treatment and Diagnosis (DCTD) to provide PD biomarker assay support for NExT drug development projects. The goal of the two programs is to develop assays that will provide robust and accurate measurements of drug effects in patient specimens using the same principles routinely applied to clinical diagnostic tests.
Imaging Program
The Cancer Imaging Program (CIP) facilities allow non-invasive evaluation of a compound’s PD profile and determination of the compound's overall biodistribution. The measurement of target inhibition in proof-of-concept animal models and first-in-human studies can facilitate decisions about the compound or trials using this compound.
Clinical Assay Laboratories
Clinical Assay Laboratories (CALs) develop assays that allow quick and accurate screening for a PD response in specimens from preclinical models and early phase clinical trial studies. Specifically, CALs develop and then validate cell-based (e.g., circulating tumor cell and tumor biopsy) and macromolecular (DNA, RNA, protein) PD assays and transfer them to clinical laboratories to support new drug evaluation in early-phase clinical trials. These assays quantify biomarkers in a variety of tissues, including tumor biopsies, blood samples, and hair follicles. CALs can also provide expertise for lead compound selection.
Development of PD assays is supported by the Pharmacodynamic Assay Development & Implementation Section (PADIS) and the National Clinical Target Validation Laboratory (NCTVL).
The role of PADIS is to develop and validate assays in clinically relevant animal models that mimic human disease and treatment. With humanized treatment and assessment conditions in animal models, the analytical performance of PD assays can be evaluated to establish assay fitness-for-purpose before use in clinical trials. As the lead compound progresses toward IND filing, validated PD assays with associated standard operating procedures (SOPs) for specimen handling, processing, and storage are then transferred to NCTVL to measure drug effects in clinical specimens. Finally, validated assays can be transferred for use in the scientific community. SOPs and information for currently available assays can be found on the DCTD Biomarkers Web site.
The NExT program in the early NExT Stage Gate will use rapid PD evaluation in animal models of human cancer to confirm that an optimized lead compound acts as intended on its molecular target. PADIS is responsible for converting discovery assays from the NExT Chemical Biology Consortium Specialized Application and Comprehensive Screening Centers into “fitness-for-purpose” PD assays; ideally, this should require only minimal adaptation. Data from such early PD studies informs go/no-go decisions on agent development within the NExT Program.
Pharmacodynamic Assay Development Pathway